Our research program in the Center for Reproductive Sciences and Department of Obstetrics, Gynecology & Reproductive Sciences studies the biological consequences of environmental chemical exposures during early pregnancy and their mechanistic links with developmental disease.Our research integrates toxicology, genomics, environmental health, computational and reproductive sciences to identify hazardous chemicals and the underlying mechanisms that contribute to disease. We utilize a variety of approaches including in vitro and computational models, omics (transcriptomic, proteomic, metabolomic and epigenomic) and functional genomics to identify mechanisms of action and biomarkers linked with environmental-disease outcomes. We are currently developing projects aiming to elucidate the relationship(s) between environmental exposures and adverse birth outcomes (e.g., neural tube defects, preterm birth, and preeclampsia). We are also very interested in elements which promote or exacerbate susceptibility (genetic factors, metabolizing enzymes, infectious disease).
Ongoing research studies:
1. Utilizing embryo models to assess chemicals for developmental toxicity.
We utilize mammalian cell/tissue based models to investigate the effects of environmental chemicals on embryogenesis.
Human embyronic stem cells (hESC) have an unlimited potential for self-renewal and the capacity to differentiate into all somatic cell types of the central nervous system. Due to their unique characteristics of being able to capture early in vivo human developmental events in vitro, hESCs can be utilized to study the effects of environmental chemicals on cellular transitions occruing during early CNS development. In our laboratory, we established a hESC model of neurogenesis to identify chemical hazards that cause developmental neurotoxicity as well as the underlying mechanisms that lead to disease (PMID: 31173147; PMID: 26827931)
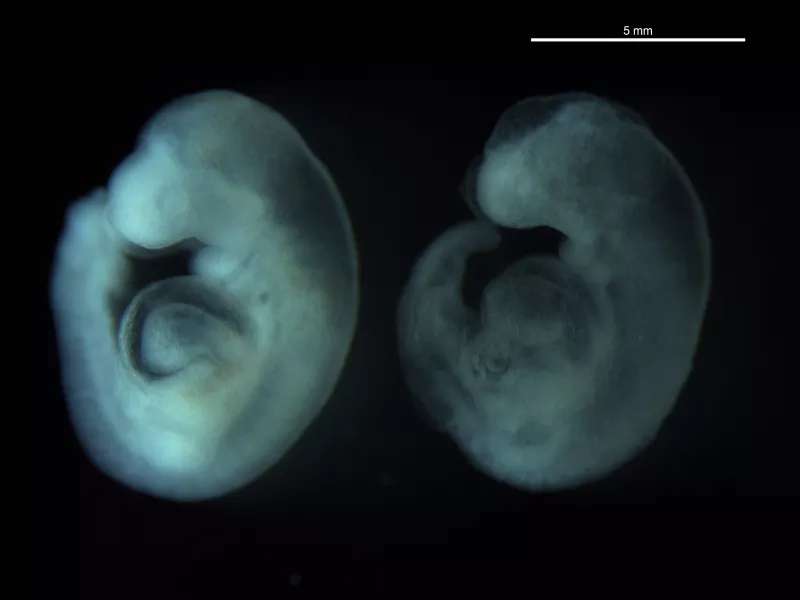
Rat post-implantation whole embryo culture (WEC) is a well-established model to evaluate environmental and genetic factors in the context of neurulation and early organogenesis. As compared to traditional rodent studies, WEC requires fewer animals (~60%), reduced experimental time and cost, and greater control over dosage and chemical specificity. Embryos are cultured on GD 10 and successfully progress through neurulation and early organogenesis over a 48h period, parallel to the transitions which occur in vivo. Morphological features of multiple organs (e.g., brain, heart, limb, limbs, eye, ear and primitive craniofacial structures) are evaluated for abnormalities or developmental delays in the context of chemical exposures. Example publications (PMID: 22664269; PMID: 22262565).
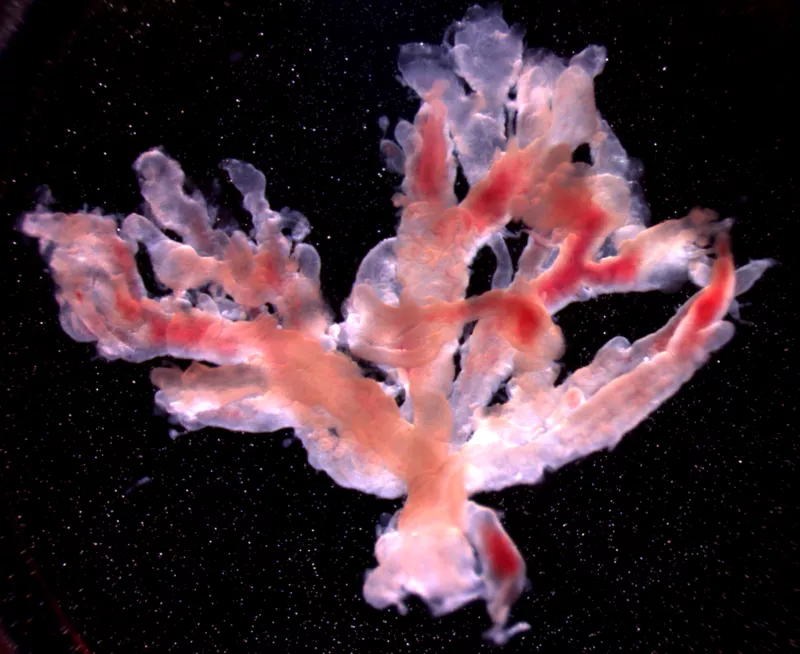
2. Using human cell/tissue models to investigate links between chemical exposures and adverse reproductive outcomes of placental origin.
The placenta acts as the critical lifeline between the maternal and fetal environments, regulating nutrients, wastes, and gases, as well as serving as a metabolic barrier from many xenobiotic insults. We study how environmental chemicals may alter placental development and underlie pregnancy complications. Utilizing primary human placental cell/tissue model systems, we are investigating the mechanistic links between environmental exposures and placental toxicity (PMID: 30202865; PMID: 28323933).
3. In vitro and in silico approaches to elucidate nuclear receptor mediated developmental toxicities.
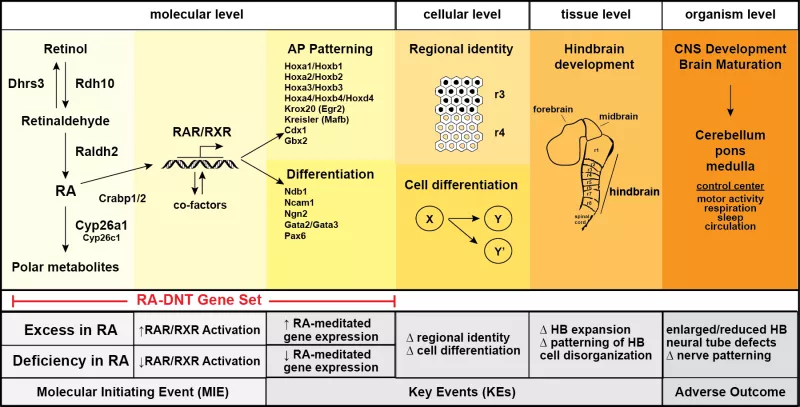
Retinoic acid (RA) is a key regulatory signaling molecule involved in multiple aspects of mammalian central nervous system (CNS) development, including hindbrain formation/patterning and neuronal differentiation. Imbalances in RA signaling pathways are linked with developmental neurotoxicity. In our laboratory, we use a combination of in vitro and in silico approaches to define conserved mechanisms critical for RA signaling and CNS development, and identify toxic chemicals that may cause harm by disrupting this pathway. (PMID: 32544423).
4. Omics and data integration
We utilize big data and omic approaches to identify signatures linked with normal development, environmental chemical exposures and disease states. We use strategies that leverage multiple datasets to define global signatures generalizable across different models or populations. These studies provide high value in understanding mechanisms that contribute to disease and assist in the identification of prognostic clinical biomarkers of exposure and disease.
5. Biomarkers and pregnancy complications
Leverage knowledge from in vitro and in vivo studies and human biological samples, we interrogate the relationship between chemical levels and morphological/molecular outcomes to assist in defining exposure and disease in the context of human pregnancy (PMID: 32493340; PMID: 29316516).